Intuitive
SharpCTMS has been designed by people actually doing your job. Handling your tasks is intuitive, and so is SharpCTMS.
Fast
Do it faster ! SharpCTMS is fast to learn, fast to use.
Sharp
Everything you need, handy, at a glance.
The advantage of an online service.
Lightweight
Use it everywhere on your computer. Every thing is in the cloud.
Reliable
Being uniquely identified, sign your actions with your electronic signature and audit them anytime.
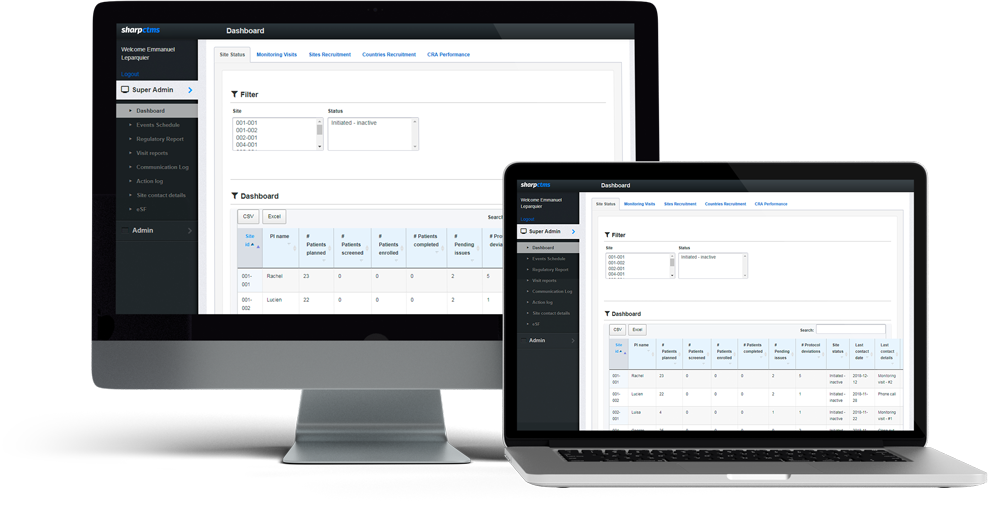
A management system adapted to your needs.
Flexible
Use it everywhere on your computer. Every thing is in the cloud.
Win-win
You only pay for what you use. The time and reliability gain for you are of more value than the CTMS cost.
Discount price on the first study.
At SharpCTMS we know the inherent difficulties of changing your way of working. Thus, since we are 100% trustful in the value of our product, we offer an immediate discount to help you start a pilot study and see how things can work in a sharper way
Request your demoFeatures
- Sites management: Manage study staff contact details, record all contacts between the Clinical Research Associate and the investigational sites, and generate data-driven reports for protocol deviations, implemented actions, SAE follow-up, etc.
- CRAs management: Handle all Clinical Research Associates’ appointments, agenda and reports easily. No more mails, all communication between Project Manager and Clinical Research Associate is set, saved and can be monitored within SharpCTMS.
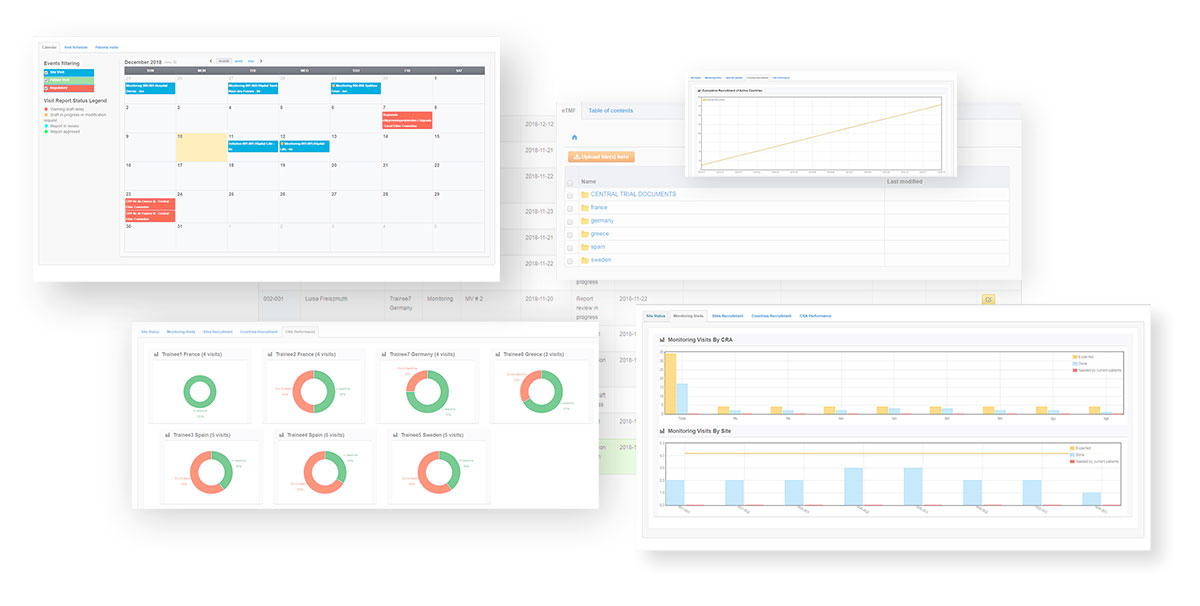
- eSite File: Upload or download all documents from the investigators sites easily for sharing and archiving, through the visit reports or directly through the DIA tree structure
- Automatic data import on demand (connection to your APIs): Imports from eCRF allow to see the next patient’s visits at a glance thanks to the automatically populated calendar.
- Audit trail: Monitor all modifications thanks to the audit trail viewable to end-users.
- Electronic reports: Compliant with FDA 21 CFR part 11 Directive about electronic records and signatures.
Who are we ?
Céline
Our clinical study expert, Céline, worked for 13 years as a CRA and as a PM but could never find a CTMS tool that was relevant. Loss of time, lack of reliability, lack of functionalities, bad design, even sometimes wrong doing, not a single CTMS she worked with could handle simply properly a study.
Emmanuel
If you can’t find something appropriate to your needs, do it yourself. Emmanuel has been a professional IT developer for 15 years. Crossing these 2 expertises gave birth to SharpCTMS in 2017. Since then, our team keeps developing and improving our CTMS to give the best experience to our users
Contact and demo
We will contact you within 48 hours !